Pipeline for chronic rhinosinusitis
Product
Candidate
Candidate
Preclinical
Phase 1
Phase 2
Phase 3
*surgically naïve ethmoid sinuses
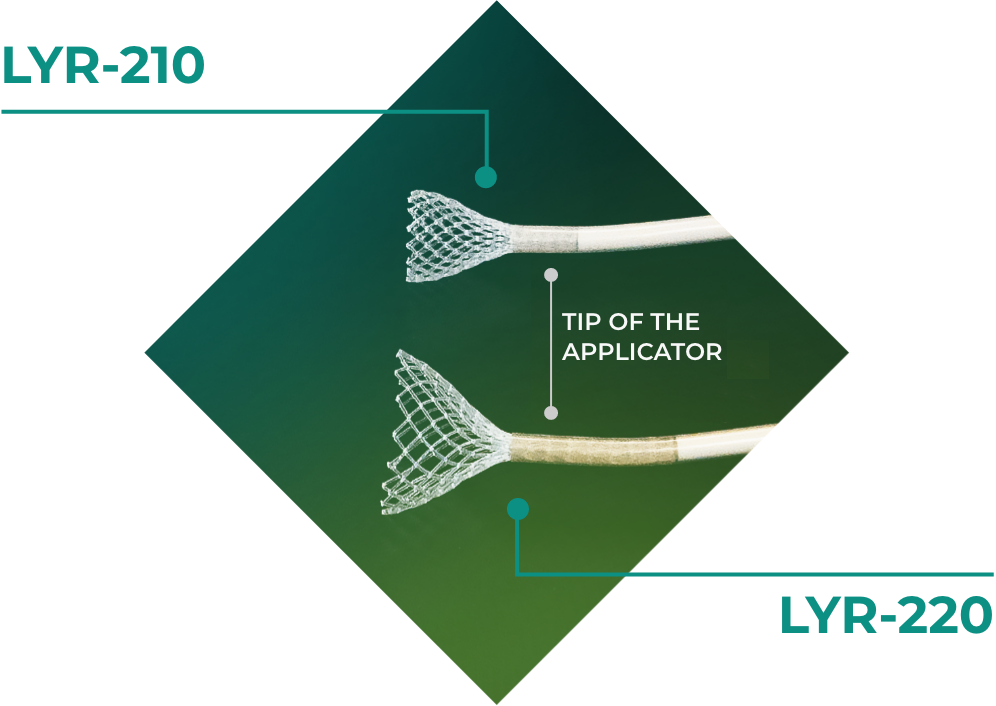
At Lyra Therapeutics, our focus is chronic rhinosinusitis (CRS). We are leveraging our innovative technology to develop new treatments for this challenging disease that impacts daily life for millions of people.
Lyra Therapeutics’ product candidates are designed to provide medicine directly to sinonasal tissues that are not accessible with conventional therapeutic approaches. Our lead product candidate, LYR-210, is in Phase 3 trials for CRS patients with surgically naïve anatomy. Our second product candidate, LYR-220, is in Phase 2 trials for CRS patients with post-surgical anatomy.


