AN INNOVATIVE THERAPEUTIC SOLUTION FOR CRS
Lyra Therapeutics’ lead product candidate, LYR-210, is in Phase 3 development for chronic rhinosinusitis (CRS) patients who have failed current treatments and require further intervention.
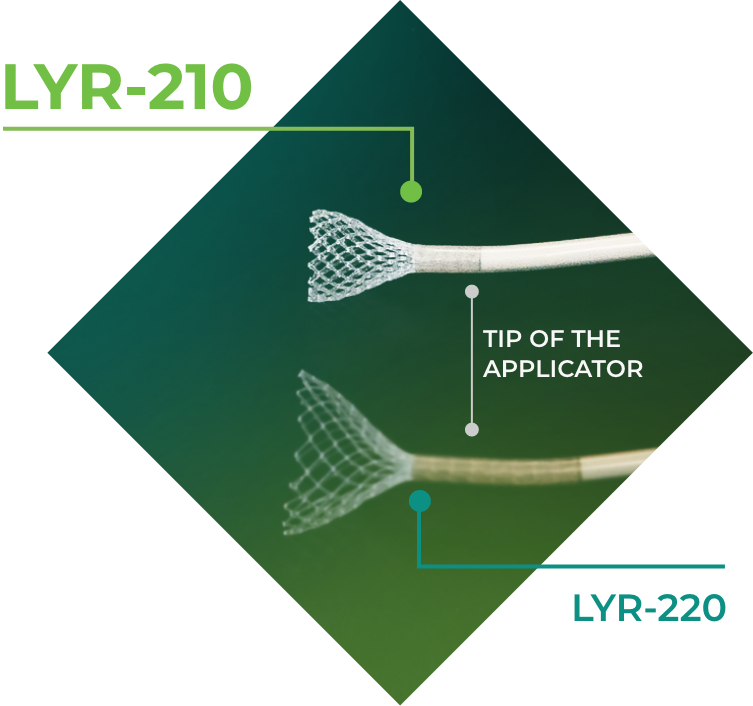
LYR-210 is a bioabsorbable nasal mesh administered in a brief in-office procedure. LYR-210 is intended to deliver up to six months of continuous, proven anti-inflammatory therapy, mometasone furoate, to the sinonasal passages to treat CRS.
LYR-210 is being evaluated in the ENLIGHTEN Phase 3 clinical program.
LYR-210 IN CLINICAL DEVELOPMENT FOR CRS
Results from the LANTERN Phase 2 randomized, controlled trial of LYR-210 for the treatment of CRS were published in the International Forum of Allergy and Rhinology.