A biocompatible mesh scaffold optimizes surface area for drug release while maintaining underlying tissue function. It is comprised of bioabsorbable polymers that are pliable to maximize patient comfort.
Lyra Therapeutics’ Technology
Medicine Where It’s Needed
Lyra Therapeutics’ proprietary technology is designed to enable sustained delivery of medication for up to six months of therapy per placement, targeting tissues deep in the sinonasal passages that are not accessible with conventional therapeutic approaches.
Lyra Therapeutics’ technology is comprised of three components that differentiate our approach for chronic rhinosinusitis (CRS):
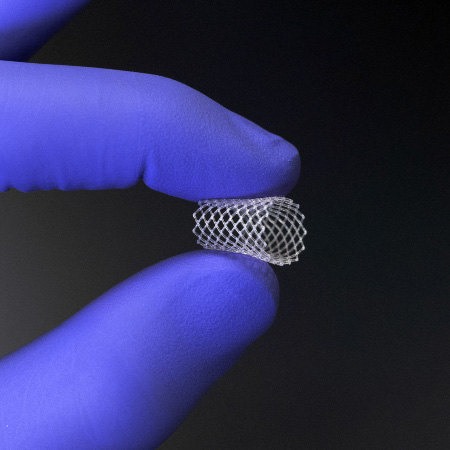

An engineered elastomeric matrix allows the product to dynamically adapt to the unique anatomy of the sinonasal passages and stay in place for up to six months.
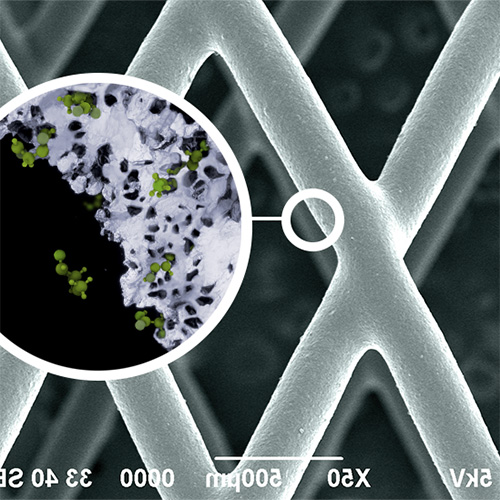
A polymer-drug formulation delivers continuous and consistent drug, improving compliance for patients.
Proprietary Technology
The Lyra Therapeutics team includes experts in materials science, drug development and drug formulation. Lyra Therapeutics has developed innovative, proprietary technology (the XTreo™ platform) that is designed to deliver medicines directly precisely and consistently to the affected tissue for sustained periods with a single administration.
Lyra Therapeutics has a robust global portfolio of intellectual property, with protection in place through 2036 and with potential additional intellectual property that would extend Lyra Therapeutics’ protection into 2042. These patents cover various aspects of Lyra Therapeutics technology, including controlled drug release and materials science for adaptive applications in biologic environments. All intellectual property protecting Lyra Therapeutics technology has been developed and is owned by the company.


